Conversion of Ores into Oxide
Conversion of Ores into Oxide: Overview
This topic covers concepts such as Calcination and Roasting and Advantages of Calcination and Roasting.
Important Questions on Conversion of Ores into Oxide
Extraction of zinc from zinc blende is achieved by
Carbon dioxide is released on heating lime stone ore, in metallurgy. This is called _____
Sulphide ore on roasting gives a gas . reacts with in the presence of activated charcoal to give . is :
Which of the following equations represents calcination ?
Give name of furnace used for calcination?
Which of the following reaction does not occur during roasting?
Aluminium is purified by :
Roasting of sulphides gives the gas as a by-product. This is a colourless gas with choking smell of burnt sulphur and causes great damage to respiratory organs as a result of acid rain. Its aqueous solution is acidic, acts as a reducing agent and its acid has never been isolated. The gas is
Heating of iron pyrites in air to remove sulphur is called
Two ores and of a metal show the following reactivity:
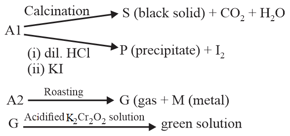
So Write the chemical formulae of and .
What is calcination? Write examples with reactions.
Explain the term: Calcination.
Explain the term:
Roasting.
What is calcination.
Partial roasting of galena gives:
is roasted to give
Choose the correct option using the code regarding roasting process.
(I) It is the process of heating the ore in air in a reverberatory furnace to obtain the oxide.
(II) It is an exothermic process.
(III) It is used for the concentration of sulphide ore.
(IV) It removes easily oxidisable volatile impurities present in the concentrated ore.
Which of the following reaction is not observed in Bessemer converter?
In the following sequence, find out the , and from the following list.
can be extracted from zinc blende by using:
